David Albert Scientific American May 94
Last Words of a Quantum Heretic John Horgan New Scientist 29 Feb 93
The keystone of Bohr's interpretation was the concept of complementarily, which held that wave-particle duality is a paradox that cannot be resolved. Bohr also ruled out the possibility that the probabilistic behaviour of quantum systems was actually the result of underlying deterministic mechanisms called hidden variables. Reality was unknowable because it was intrinsically indefinite, Bohr insisted.
Particles are always particles
In trying to explain Bohr's approach, Bohm became dissatisfied with it. "The whole idea of science so far has been to say that underlying the phenomenon is some reality which explains things," he explained. "It was not that Bohr denied reality, but he said quantum mechanics implied there was nothing more that could be said about it." Such a view, Bohm decided, reduced quantum mechanics to "a system of formulas that we use to make predictions or to control things technologically. I said, that's not enough. I don't think I would be very interested in science if that were afl there was." In 1952 Bohm defied Bohr's prohibition against hidden-variable explanations in a classic two-part paper in Physical Review entitled "A suggested interpretation of the quantum theory in terms of 'hidden' variables". He proposed that particles are indeed particles-and at afl times, not just when they are observed. Their behaviour is determined by an unusual field or wave consisting both of classical versions of forces such as electromagnetism and an entirely new force-which Bohm called the quantum potential-that is responsible for nonclassical effects. The positions of particles in tum serve as the hidden variables determining the nature of the pilot wave. Bohm's interpretation was causal, or deterministic. Particles always had a distinct position and velocity, but any effort to measure these properties precisely would destroy information about them by physically altering the pilot wave. Bohm gave the uncertainty principle a purely physical rather than metaphysical meaning. Bohr had interpreted the uncertainty pn'nciple, Bohm explained, as meaning "not that there is uncertainty, but that there is an inherent ambiguity" in a quantum sv'tem. Bohm sent out preprints of the paper and was quickly informed that his interpretation was an old one, proposed 25 years earlier by Louis de Broglie. De Broglie had abandoned the pilot-wave concept after Wolfgang Pauli pointed out that, when applied to systems involving more than one particle, it led to "some very strange behaviour" This strange behaviour referred to by Pauli, Bohm realised, was nonlocality. Actually, nonlocality was a feature intrinsic to all quantum theories, not just Bohm's. Einstein had demonstrated this fact back in 1935 in an effort to show that quantum mechanics must be flawed. Working together with Boris Podolsky and Nathan Rosen at Princeton, Einstein proposed a thought experiment involving rwo particles that spring from a common source and fly in opposite directions. According to the standard model of quantum mechanics, neither particle has a definite position or momentum before it is measured; but by measuring the momentum of one particle, the physicist instantaneously forces the other particle to assume a fixed position-even if it is on the other side of the Galaxy. Deriding this effect as "spooky action at a distance", Einstein argued that it violated both common sense and the theory of relativity, which prohibits the propagation of effects faster than the speed of light-I quantum mechanics must be an incomplete theory. Perhaps because he had always had a holistic view of reality, Bohm was not disturbed by nonlocality. "I must have tacitly been feeling all along that quantum mechanics was nonlocal," he said. In Quantum Theory, Bohm even suggested an experiment that could demonstrate nonlocaliry more clearly and easily than the one proposed by Einstein, Podoisky and Rosen. Bohm called for measuring not the momentum and position of rwo particles from a common source but rather their spin. Bohm's spin experiment became the basis for a brilliant mathematical proof bv Bell in 1964 showing that no local hidden-variable theory could replicate the predictions of quantum mechanics. In 1982, a group led bN, the French physicist Alain Aspect at the University of Paris-South, carried out Bohm's experiment, demonstrating once and for all that quantum mechanics does indeed require spooky action. (The reason that nonlocality does not violate the theory of relativity is that one cannot exploit it to transmit infon-nation faster than light or instantaneously.) Bohm said he never had any doubts about the outcome of the experiment: 'it would have been a terrific surprise to find out otherwise." Ironically, Bell's theorem and the Aspect experiment were widely thought to rule out all hidden-variable theories, including Bohm's. It was Bell who pointed out years later that Bohm's theory, since it was nonlocal, was not ruled out by his theorem. According to Bohm's model, nonlocality was mediated through the pilot wave: any localised physical act, such as the measurement of a particle, would instantaneously alter the shape of the entire pilot wave, affecting all particles under its influence. Bohm continued to develop the pilot-wave theory through the 1980s with the help of collaborators such as Hiley. In its latest version, the Bohmian pilot wave is quite distinct from the one posited by de Broglie. De Broglie conceived of the pilot wave as a kind of mechanical force which pushed particles this way and that through the transmission of energy. Bohm's pilot wave is more subtle: it guides particles not through its amplitude but through its form-much as the form rather than the amplitude of a flight-controller's radio transmission controls a plane's behaviour. The wave's abuity to influence particles therefore does not diminish with distance, as classical waves do. In the last decade, Bohm also became absorbed in another perennial puzzle: why quantum effects are generally lim ited to very small-scale phenomena. Two recent efforts to explain this mystery left him unimpressed. One of these, proposed by Gian Carlo Ghirardi of the University of Trieste and others, holds that as a quantum entity propagates through space, its multiple, possible states converge into a single state that behaves in a classical way. Roger Penrose of the University of Oxford presented another possibility in his book The Emperor's New Mind: quantum effects disappear in systems containing so much mass that gravity-which is usually negligible at subatomic scales-becomes a factor. Bohm favoured what he felt was a much simpler explanation: heat. Various lines of evidence-notably the fact that superconductivity, which relies on quantum effects, occurs only at very low temperatures-suggest that thermal energy swamps quantum effects. To completely resolve the issue of the limits of quantum effects, Bohm contended that: "It would be required to connect thermodynamics and quantum mechanics in a deep ftindamental way rather than the present superficial way, which is that you start with quantum mechanics and then apply statistics. It may be that thermal properties are just as essential as quantum properties, or there's something deeper than both." To arrive at such a theory, physicists might need to jettison some basic assumptions about the organisation of nature. "Fundamental notions like order and structure condition our thinking unconsciously, and new kinds of theories depend on new kinds of order," he said. During the Enlightenment, he noted, thinkers such as Rene Descartes and Isaac Newton replaced the ancients' concept of order with a mechanistic view. Although the advent of relativity and other theories has brought about modifications in this order, Bohm said, "the basic idea is still the same: a mechanical order described by coordinates". Bohm himself began formulating what he called the implicate order several decades ago. His ideas were inspired in part by a simple experiment he saw on television, in which a drop of ink was squeezed onto a cylinder of glycerine. When the cylinder rotated, the ink diffused through the glycerine in an apparently irreversible fashion; its order seemed to have disintegrated. But when the direction of rotation was reversed, the ink gathered into a drop again. Bohm made this simple experiment into a metaphor for au of reality. Underlying the apparently chaotic realm of physical appearances-the explicate order-there is always a deeper, implicate order that is often hidden. Applying this concept to the quantum realm, Bohm proposed that the implicate order is the quantum potential, a field consisting of an infinite number of fluctuating waves. The overlapping of these waves generates what appear to us as particles: these constitute the explicate order. Even such seemingly fundamental conceprs as space and time may be merely explicate manifestations of some "nonlocal, deeper implicate order', according to Bohm. Bohm hoped the implicate order could even point the way to a resolution of that perennial conundrum of philosophy, the mind-matter problem. His belief was based on hints and rough analogies rather than on any concrete evidence. For example, he compared the way a pilot wave guides a particle to the way thought guides Ehe movements of a dancer. "The movement of the body is coming from thought, and the movement of the eleciron Ls coming from something very subtle, this wave. So there are similarities, which should make it possible to relate them."

Krishnamurti and David Bohm discuss the observer and the observed.
John Horgan
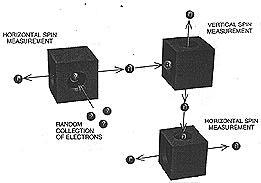
Spin behaviour is disrupted in a sequence ofthree measurementsElectrons are measured one at a time for their hrizontal spins (left), then for their vertical spins (right) and again for their horizontal spins (bottom). The vertical box disrupts the spin of half those electrons, so that half emerge from the second horizontal box with right spin and half with left.

Spin equivalent of two-slit interference: Two-path spin detection experiment depicts the unusual spin behaviour of electrons. On the left diagram right-spinning electrons are reflected back from an up down detector and recohered. The subsequent horizontal detector confirms they are all right spinning. However if one of the paths (top) are obstructed, then only 50% of the electrons subsequently measure as right spinning. This is because those which make it to the end definitely were down spinning and hence had had a disrupting measurement made of their vertical spin.
Hence, Bohm's theory accounts for all the unfathomable-looking behaviors of electrons discussed earlier every bit as well as the standard interpretation does. Moreover, and this point is important, ft is free of any of the metaphysical perplexties associated with quantum-mechanical -perposition.

Left: A graphical depiction of the quantum potential.
Right: Trajectories of an electron in a double slit experiment.
The first is a deterministic law (namely, Schödinger's equation) that describes how the wave functions of physical systems evolve over time. lt is:
The second element is a deterministic law of the motions of the particles:
The third element Is a statistical rule analogous to one used In classical statistical mechanics. It stipulates precisely how one goes about 'averaging over' one's Inevitable Ignorance of the exact states of physical systems. It runs as follows. Assume one is given the wave function of a certain system but no information about the positions of its particles. To calculate the motions of those particles in the future, what one ought to suppose is that the probability that those particles are currently located at some position (X1 ... X3N) is equal to
No comments:
Post a Comment